The atmosphere is a mixture of various gases. It extends from the Earth’s surface to an altitude of 900 km, protecting the planet from the harmful spectrum of solar radiation, and contains the gases necessary for all life on the planet. The atmosphere retains solar heat, heating the air near the earth’s surface and creating a favorable climate.
Atmospheric composition
The Earth’s atmosphere consists mainly of two gases - nitrogen (78%) and oxygen (21%). In addition, it contains impurities of carbon dioxide and other gases. in the atmosphere exists in the form of steam, droplets of moisture in the clouds and ice crystals.
Atmosphere layers
The atmosphere consists of many layers, between which there are no clear boundaries. The temperatures of the different layers differ markedly from each other.
Airless magnetosphere. Here most of the Earth’s satellites are flying outside the earth’s atmosphere. Exosphere (450-500 km from the surface). Almost does not contain gases. Some weather satellites fly in the exosphere. The thermosphere (80–450 km) is characterized by high temperatures, reaching 1700 ° C in the upper layer. Mesosphere (50-80 km). In this sphere falls as the height increases. It is here that most of the meteorites (fragments of cosmic rocks) that enter the atmosphere burn down. Stratosphere (15-50 km). Contains ozone, i.e., a layer of ozone that absorbs the ultraviolet radiation of the sun. This leads to an increase in temperature near the surface of the Earth. Jet planes usually fly here because the visibility of this word is very good and almost no interference due to weather conditions. Troposphere. The altitude varies from 8 to 15 km from the earth's surface. This is where the weather of the planet is formed, as in this layer contains the most water vapor, dust and winds. The temperature decreases with distance from the earth's surface.
Atmosphere pressure
Although we do not feel this, the layers of the atmosphere put pressure on the surface of the Earth. The highest atmospheric pressure is near the surface, and as it moves away from it, it gradually decreases. It depends on the temperature of the land and ocean, and therefore in areas at the same height above sea level, there is often a different pressure. Low pressure brings wet weather, and when high pressure usually sets clear weather.
The movement of air masses in the atmosphere
Changes in temperature and pressure cause the atmosphere to mix in the lower layers of the atmosphere. This is how the winds blow from high pressure areas to low ones. In many regions, there are local winds caused by changes in land and sea temperatures. Mountains also have a significant influence on the direction of the winds.
Greenhouse effect
 Carbon dioxide and other gases that are part of the earth's atmosphere, retain solar heat. This process is called the greenhouse effect, as it is in many ways reminiscent of the circulation of heat in greenhouses. Greenhouse effect entails global warming on the planet. In areas of high pressure - anticyclones - clear sunny weather sets. In areas of low pressure - cyclones - weather is usually unstable. Heat and light entering the atmosphere. Gases trap heat reflected from the earth’s surface, thereby causing a rise in temperature on Earth.
Carbon dioxide and other gases that are part of the earth's atmosphere, retain solar heat. This process is called the greenhouse effect, as it is in many ways reminiscent of the circulation of heat in greenhouses. Greenhouse effect entails global warming on the planet. In areas of high pressure - anticyclones - clear sunny weather sets. In areas of low pressure - cyclones - weather is usually unstable. Heat and light entering the atmosphere. Gases trap heat reflected from the earth’s surface, thereby causing a rise in temperature on Earth.
In the stratosphere, there is a special ozone layer. Ozone detains most of the ultraviolet radiation of the Sun, protecting the Earth and all life on it from it. Scientists have found that the cause of the destruction of the ozone layer are special chlorofluorocarbon gases contained in some aerosols and refrigeration equipment. 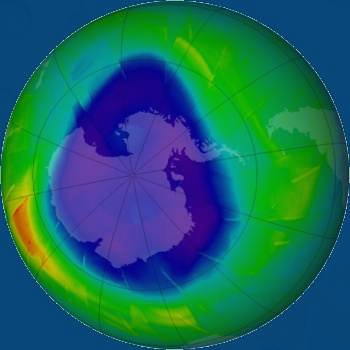 Over the Arctic and Antarctica, huge holes were discovered in the ozone layer, contributing to an increase in the amount of ultraviolet radiation acting on the surface of the Earth.
Over the Arctic and Antarctica, huge holes were discovered in the ozone layer, contributing to an increase in the amount of ultraviolet radiation acting on the surface of the Earth.
Ozone is formed in the lower atmosphere as a result of a chemical reaction between solar radiation and various exhaust fumes and gases. It is usually dispersed throughout the atmosphere, but if a closed layer of cold forms under the warm air, ozone concentrates and smog occurs. Unfortunately, this cannot compensate for the loss of ozone in ozone holes.
The photograph from the satellite clearly shows a hole in the ozone layer over Antarctica. The size of the hole varies, but scientists believe that it is constantly increasing. Attempts are being made to reduce the level of exhaust gases in the atmosphere. Air pollution should be reduced and smokeless fuels should be used in cities. Smog causes eye irritation and choking in many people.
The origin and evolution of the Earth’s atmosphere
The modern atmosphere of the Earth is the result of a long evolutionary development. It arose as a result of the joint action of geological factors and the vital activity of organisms. Throughout its geological history, the earth's atmosphere has undergone several deep reconstructions. Based on geological data and theoretical (prerequisites, the pristine atmosphere of the young Earth, which existed about 4 billion years ago, could consist of a mixture of inert and noble gases with a small addition of passive nitrogen (N. A. Yasamanov, 1985; A. S. Monin, 1987; O. G. Sorokhtin, S. A. Ushakov, 1991, 1993). Currently, the view on the composition and structure of the early atmosphere has changed somewhat. The primary atmosphere (protoatmosphere) at the earliest protoplanetary stage., Ie, older than 4.2 billion years old, could consist of a mixture of methane, ammonia and carbon As a result of degassing of the mantle and active weathering processes occurring on the earth’s surface, water vapor, carbon compounds in the form of CO 2 and CO, sulfur and its compounds, as well as strong halogen acids — HCI, HF, HI and boric acid that were supplemented with methane, ammonia, hydrogen, argon, and some other noble gases in the atmosphere.This primary atmosphere was extremely thin. Therefore, the temperature at the earth's surface was close to the temperature of radiative equilibrium (A. S. Monin, 1977).
With the passage of time, the gas composition of the primary atmosphere under the influence of weathering of rocks that protruded on the earth's surface, the activity of cyanobacteria and blue-green algae, volcanic processes and the action of sunlight began to transform. This led to the decomposition of methane into hydrogen and carbon dioxide, ammonia - into nitrogen and hydrogen; carbon dioxide, which slowly descended to the earth's surface, and nitrogen began to accumulate in the secondary atmosphere. Owing to the vital activity of blue-green algae, during photosynthesis, oxygen was produced, which, however, at the beginning was mainly spent on “oxidation of atmospheric gases, and then rocks. In this case, ammonia, oxidized to molecular nitrogen, began to accumulate intensively in the atmosphere. It is assumed that a significant amount of nitrogen in the modern atmosphere is relict. Methane and carbon monoxide were oxidized to carbon dioxide. Sulfur and hydrogen sulfide were oxidized to SO 2 and SO 3, which, due to their high mobility and lightness, quickly escaped from the atmosphere. Thus, the atmosphere from the reducing, as it was in the Archean and Early Proterozoic, gradually turned into oxidizing.
Carbon dioxide entered the atmosphere as a result of the oxidation of methane, and as a result of the degassing of the mantle and the weathering of rocks. In the event that all the carbon dioxide released in the entire history of the Earth was preserved in the atmosphere, its partial pressure could now become the same as on Venus (O. Sorokhtin, S. A. Ushakov, 1991). But on Earth there was a reverse process. A significant part of carbon dioxide from the atmosphere was dissolved in the hydrosphere, in which it was used by hydrobionts to build its shell and biogenically turned into carbonates. Later on, the most powerful strata of chemogenic and organogenic carbonates were formed.
Oxygen entered the atmosphere from three sources. For a long time, starting from the moment of the Earth’s appearance, it was released during the degassing of the mantle and was mainly spent on oxidation processes. Another source of oxygen was the photodissociation of water vapor by hard ultraviolet solar radiation. Appearances; free oxygen in the atmosphere led to the death of most prokaryotes, who lived in reducing conditions. Prokaryotic organisms have changed their habitat. They left the Earth’s surface to its depths and areas where restoration conditions still remained. They were replaced by eukaryotes, which began to energetically convert carbon dioxide into oxygen.
During the Archaean and a significant part of the Proterozoic, almost all of the oxygen that arises both: by abiogenic and biogenic, was mainly spent on the oxidation of iron and sulfur. By the end of the Proterozoic, all metallic ferrous iron, which was on the earth's surface, either oxidized or moved into the earth's core. This led to the fact that the partial pressure of oxygen in the Early Proterozoic atmosphere has changed.
In the middle of the Proterozoic, the oxygen concentration in the atmosphere reached the Yuri point and was 0.01% of the current level. From this time on, oxygen began to accumulate in the atmosphere and, probably, at the end of the Riphean, its content reached the Pasteur point (0.1% of the current level). Possibly, in the Vendian period, the ozone layer arose and never disappeared in this time period.
The appearance of free oxygen in the earth's atmosphere stimulated the evolution of life and led to the emergence of new forms with a more advanced metabolism. If earlier eukaryotic single-celled algae and cyaneas, which appeared at the beginning of the Proterozoic, required only 10 -3 of its oxygen concentration in water, then with the appearance of skeleton-free Metazoa at the end of the early Vendian, i.e. about 650 million years ago, the oxygen concentration in the atmosphere would have to be much higher. After all, Metazoa used oxygen respiration and for this it was required that the partial pressure of oxygen reached a critical level - Pasteur's point. In this case, the anaerobic fermentation process was replaced by an energetically more promising and progressive oxygen metabolism.
After that, further accumulation of oxygen in the earth's atmosphere occurred fairly quickly. A progressive increase in the volume of blue-green algae contributed to the achievement in the atmosphere of the oxygen level necessary for the life support of the animal world. A certain stabilization of the oxygen content in the atmosphere occurred from the moment when the plants reached the land, about 450 million years ago. The emergence of plants on land, which occurred in the Silurian period, led to the final stabilization of the oxygen level in the atmosphere. From this time on, his concentration began to fluctuate within rather narrow limits, never going beyond the framework of the existence of life. Fully oxygen concentration in the atmosphere has stabilized since the appearance of flowering plants. This event occurred in the middle of the Cretaceous, i.e. about 100 million years ago.
The bulk of the nitrogen formed on early stages development of the Earth, mainly due to the decomposition of ammonia. With the advent of organisms, the process of binding atmospheric nitrogen into organic matter and its burial in marine sediments began. After the release of organisms on land, nitrogen became buried in continental sediments. The processing of free nitrogen has especially increased with the advent of land plants.
At the turn of cryptozoic and Phanerozoic, that is, about 650 million years ago, the content of carbon dioxide in the atmosphere decreased to tenths of a percent, and the content close to the current level, it reached only recently, approximately 10-20 million years ago.
Thus, the gas composition of the atmosphere not only provided the organisms with living space, but also determined the peculiarities of their vital activity, promoted resettlement and evolution. The resulting disruptions in the distribution of the atmospheric gas composition favorable for organisms, due to both cosmic and planetary reasons, led to mass extinctions of the organic world, which occurred repeatedly during the cryptosis and at certain frontiers of the Phanerozoic history.
Ethnospheric functions of the atmosphere
The Earth's atmosphere provides the necessary substance, energy and determines the direction and speed of metabolic processes. The gas composition of the modern atmosphere is optimal for the existence and development of life. Being an area of weather and climate, the atmosphere should create a comfortable environment for people, animals and vegetation. Deviations in one direction or the other as atmospheric air and weather conditions create extreme conditions for the life activity of the animal and plant world, including for humans.
The Earth's atmosphere not only provides the conditions for the existence of mankind, being the main factor in the evolution of the ethnosphere. At the same time, it turns out to be an energy and raw material resource for production. In general, the atmosphere is a factor that preserves human health, and some areas, due to their physical-geographical conditions and air quality, serve as recreational areas and are areas intended for sanatorium-resort treatment and recreation of people. Thus, the atmosphere is a factor of aesthetic and emotional impact.
The ethnospheric and technospheric functions of the atmosphere, which were defined quite recently (E. D. Nikitin, N. A. Yasamanov, 2001), need independent and in-depth research. So, the study of energetic atmospheric functions is very topical both in terms of the occurrence and operation of processes detrimental to the environment, and in terms of the impact on human health and well-being. In this case we are talking about the energy of cyclones and anticyclones, atmospheric vortices, atmospheric pressure and other extreme atmospheric phenomena, the effective use of which will contribute to the successful solution of the problem of obtaining alternative sources of energy that do not pollute the environment. After all, the air environment, especially that part of it, which is located above the World Ocean, is an area of release of a huge amount of free energy.
For example, it has been established that tropical cyclones of average strength produce only energy per day equivalent to 500 thousand atomic bombs dropped on Hiroshima and Nagasaki. For 10 days of the existence of such a cyclone, energy is released, sufficient to meet all the energy needs of a country like the United States for 600 years.
In recent years, a large number of works by scientists in the natural sciences have been published, in one way or another relating to various aspects of the activity and the influence of the atmosphere on terrestrial processes, which indicates the intensification of interdisciplinary interactions in modern natural science. At the same time, the integrating role of certain of its areas is manifested, among which the functional-ecological direction in geo-ecology should be noted.
This direction stimulates the analysis and theoretical synthesis of information on environmental functions and the planetary role of various geospheres, and this, in turn, is an important prerequisite for the development of methodology and scientific foundations of the holistic study of our planet, rational use and protection of its natural resources.
The Earth’s atmosphere consists of several layers: the troposphere, the stratosphere, the mesosphere, the thermosphere, the ionosphere, and the exosphere. In the upper part of the troposphere and the lower part of the stratosphere is a layer enriched in ozone, called the ozone screen. Established certain (daily, seasonal, annual, etc.) patterns in the distribution of ozone. Since its inception, the atmosphere affects the course of planetary processes. The primary composition of the atmosphere was completely different than at present, but over time, the proportion and role of molecular nitrogen grew steadily, free oxygen appeared about 650 million years ago, the amount of which continuously increased, but the concentration of carbon dioxide decreased accordingly. The high mobility of the atmosphere, its gas composition and the presence of aerosols determine its prominent role and active participation in a variety of geological and biospheric processes. The role of the atmosphere is great in the redistribution of solar energy and the development of catastrophic natural phenomena and disasters. Atmospheric eddies — tornadoes, hurricanes, typhoons, cyclones and other phenomena have a negative impact on the organic world and natural systems. The main sources of pollution along with natural factors are various forms of human economic activity. Anthropogenic effects on the atmosphere are expressed not only in the appearance of various aerosols and greenhouse gases, but also in an increase in the amount of water vapor, and are manifested in the form of smog and acid rain. Greenhouse gases change the temperature regime of the earth's surface, emissions of some gases reduce the volume of the ozone screen and contribute to the occurrence of ozone holes. The ethnospheric role of the Earth’s atmosphere is great.
The role of the atmosphere in natural processes
The surface atmosphere, due to its intermediate state between the lithosphere and outer space and its gas composition, creates conditions for the vital activity of organisms. At the same time, weathering and the intensity of rock destruction, transport and accumulation of detrital material depend on the amount, nature and frequency of precipitation, on the frequencies and strength of winds and especially on air temperature. The atmosphere is a central component of the climate system. Temperature and humidity of the air, clouds and precipitation, wind - all this characterizes the weather, that is, the continuously changing state of the atmosphere. At the same time, these same components also characterize the climate, i.e., the averaged long-term weather regime.
The composition of gases, the presence of clouds and various impurities, which are called aerosol particles (ash, dust, water vapor particles), determine the peculiarities of the passage of solar radiation through the atmosphere and impede the departure of thermal radiation of the Earth into space.
The atmosphere of the Earth is very mobile. The processes and changes in its gas composition, thickness, clouds, transparency and the presence of certain aerosol particles in it affect both the weather and the climate.
The action and direction of natural processes, as well as life and activity on Earth are determined by solar radiation. It gives 99.98% of the heat entering the earth's surface. Every year it is 134 * 1019 kcal. This amount of heat can be obtained by burning 200 billion tons of coal. The supply of hydrogen that creates this flow of thermonuclear energy in the mass of the Sun is enough for at least another 10 billion years, i.e. for a period twice as large as our planet itself exists.
About 1/3 of the total amount of solar energy entering the upper boundary of the atmosphere is reflected back into world space, 13% is absorbed by the ozone layer (including almost all ultraviolet radiation) ,. 7% - the rest of the atmosphere and only 44% reaches the earth's surface. The total solar radiation reaching the Earth in 24 hours is equal to the energy that mankind received as a result of burning all types of fuel over the last millennium.
The number and nature of the distribution of solar radiation on the earth's surface are closely dependent on cloudiness and transparency of the atmosphere. The height of the sun above the horizon, the transparency of the atmosphere, the content of water vapor, dust, total carbon dioxide, etc., affect the amount of diffuse radiation.
The maximum amount of diffuse radiation falls into the polar regions. The lower the sun above the horizon, the less heat comes to this area.
Of great importance are the transparency of the atmosphere and cloudiness. On a cloudy summer day it is usually colder than on a clear day, as daytime clouds prevent the earth’s surface from heating.
A large role in the distribution of heat is played by the dustiness of the atmosphere. Fine dust and ash particles in it, which affect its transparency, adversely affect the distribution of solar radiation, most of which is reflected. Fine particles enter the atmosphere in two ways: this is either ash emitted during volcanic eruptions, or desert dust carried by winds from arid tropical and subtropical areas. Especially a lot of such dust is formed in the period of droughts, when it is carried by streams of warm air into the upper layers of the atmosphere and is able to stay there for a long time. After the eruption of the Krakatau volcano in 1883, the dust emitted tens of kilometers into the atmosphere was in the stratosphere for about 3 years. As a result of the eruption in 1985 of the El Chichon volcano (Mexico), dust reached Europe, and therefore there was a slight decrease in surface temperatures.
Earth's atmosphere contains a variable amount of water vapor. In absolute terms, by mass or volume, its amount is from 2 to 5%.
Water vapor, like carbon dioxide, increases the greenhouse effect. In the clouds and fogs occurring in the atmosphere, peculiar physicochemical processes take place.
The primary source of water vapor into the atmosphere is the surface of the oceans. A layer of water with a thickness of 95 to 110 cm evaporates annually from it. Part of the moisture returns to the ocean after condensation, and another is directed by air streams towards the continents. In areas of a variable-humid climate, precipitation moistens the soil, and in humid areas they create groundwater reserves. Thus, the atmosphere is a battery of moisture and a reservoir of sediment. and fogs forming in the atmosphere provide moisture for the soil cover and thus play a decisive role in the development of the animal and plant world.
Atmospheric moisture is distributed over the earth's surface due to the mobility of the atmosphere. It has a very complex system of winds and pressure distribution. Due to the fact that the atmosphere is in continuous motion, the nature and extent of the distribution of wind currents and pressures change all the time. Circulation scales vary from micrometeorological, only a few hundred meters in size, to a global one - in several tens of thousands of kilometers. Huge atmospheric vortices are involved in creating systems of large-scale air currents and determine the general circulation of the atmosphere. In addition, they are sources of catastrophic atmospheric phenomena.
The distribution of weather and climatic conditions and the functioning of living matter depend on atmospheric pressure. In that case, if the atmospheric pressure fluctuates in small limits, it does not play a decisive role in human well-being and animal behavior and does not affect the physiological functions of plants. Frontal phenomena and weather changes are usually associated with changes in pressure.
Atmospheric pressure is of fundamental importance for the formation of wind, which, being a relief-forming factor, has the strongest effect on the animal and plant world.
The wind can suppress plant growth and at the same time promotes seed transport. The role of wind in the formation of weather and climatic conditions. He also acts as a regulator of sea currents. Wind as one of the exogenous factors contributes to the erosion and deflation of weathered material over long distances.
Ecological and geological role of atmospheric processes
Reducing the transparency of the atmosphere due to the appearance of aerosol particles and solid dust in it affects the distribution of solar radiation, increasing the albedo or reflectivity. Various chemical reactions that cause ozone decomposition and the generation of “mother-of-pearl” clouds consisting of water vapor lead to the same result. Global change reflectivity, as well as changes in the gas composition of the atmosphere, mainly greenhouse gases, cause climate change.
Uneven heating, causing differences in atmospheric pressure over different parts of the earth's surface, leads to atmospheric circulation, which is a distinctive feature of the troposphere. When a difference in pressure occurs, it rushes from areas of high pressure to areas of low pressure. These movements of air masses along with humidity and temperature determine the main ecological and geological features of atmospheric processes.
Depending on the speed, the wind produces various geological work on the earth's surface. At a speed of 10 m / s, it pumps thick branches of trees, picks up and carries dust and fine sand; at a speed of 20 m / s, it breaks down tree branches, carries sand and gravel; at a speed of 30 m / s (storm) it tears off the roofs of houses, tears up trees by its roots, breaks pillars, moves pebbles and carries fine rubble, and a hurricane wind at 40 m / s destroys houses, breaks down and tears down power transmission poles large trees.
Squall storms and tornadoes (tornadoes) have a large negative environmental impact with catastrophic consequences — atmospheric eddies that arise in the warm season on powerful atmospheric fronts that have a speed of up to 100 m / s. Squalls are horizontal whirlwinds with hurricane wind speeds (up to 60-80 m / s). They are often accompanied by heavy rains and thunderstorms lasting from a few minutes to half an hour. Squalls cover areas up to 50 km wide and cover a distance of 200-250 km. A squall storm in Moscow and the Moscow region in 1998 damaged the roofs of many houses and threw trees.
Tornadoes, called tornadoes in North America, are powerful, funnel-like atmospheric eddies, often associated with thunderclouds. These are air columns tapering in the middle with a diameter from several tens to hundreds of meters. The tornado has the appearance of a funnel, very similar to the trunk of an elephant, descending from the clouds or rising from the surface of the earth. Possessing a strong rarefaction and high speed of rotation, the tornado travels up to several hundred kilometers, drawing in dust, water from reservoirs, and various objects. Powerful tornadoes are accompanied by thunder, rain and have great destructive power.
Tornadoes rarely occur in subpolar or equatorial areas where it is constantly cold or hot. There are few tornadoes in the open ocean. Tornadoes occur in Europe, Japan, Australia, the USA, and in Russia they are especially frequent in the Central Black Earth region, in Moscow, Yaroslavl, Nizhny Novgorod and Ivanovo regions.
Tornadoes raise and move cars, houses, cars, bridges. Especially destructive tornadoes (tornadoes) are observed in the USA. From 450 to 1500 tornadoes are marked annually, with an average number of 100 victims. Tornadoes are fast catastrophic atmospheric processes. They are formed in just 20-30 minutes, and their existence time is 30 minutes. Therefore, to predict the time and place of occurrence of tornadoes is almost impossible.
Other destructive, but atmospheric vortices operating for a long time are cyclones. They are formed due to the pressure drop, which in certain conditions contributes to the occurrence of circular movement of air flow. Atmospheric vortices originate around powerful ascending flows of moist warm air and rotate clockwise in high speed in the southern hemisphere and counterclockwise in the northern. Cyclones, unlike tornadoes, originate over the oceans and produce their destructive actions over the continents. The main destructive factors are strong winds, intense precipitation in the form of snowfall, heavy rains, hail and surge floods. Winds with speeds of 19–30 m / s form a storm, 30–35 m / s make a storm, and more than 35 m / s make a hurricane.
Tropical cyclones - hurricanes and typhoons - have an average width of several hundred kilometers. Wind speed inside the cyclone reaches hurricane force. Tropical cyclones last from several days to several weeks, moving at a speed of 50 to 200 km / h. Mid-latitude cyclones have a larger diameter. Their transverse dimensions range from one thousand to several thousand kilometers, the wind speed is stormy. They move in the northern hemisphere from the west and are accompanied by hail and snowfall that are catastrophic. In terms of the number of victims and the damage caused, cyclones and associated hurricanes and typhoons are the largest atmospheric phenomena after floods. In densely populated areas of Asia, the number of victims during hurricanes is measured in thousands. In 1991, 125 thousand people died in Bangladesh during a hurricane that caused the formation of 6-meter-high sea waves. Great damage caused by typhoons in the United States. At the same time dozens and hundreds of people die. In Western Europe, hurricanes cause less damage.
Thunderstorms are considered a catastrophic atmospheric phenomenon. They occur with very fast lifting of warm, moist air. On the border of the tropical and subtropical zones, thunderstorms occur 90-100 days a year, in the temperate zone 10-30 days. In our country, the most thunderstorms occur in the North Caucasus.
Thunderstorms usually last less than an hour. Of particular danger are intense downpours, hailstorms, lightning strikes, wind gusts, vertical air currents. Danger of hail is determined by the size of hailstones. In the North Caucasus, the mass of hailstones once reached 0.5 kg, while in India, hailstones with a mass of 7 kg were noted. The most hazardous areas in our country are located in the North Caucasus. In July 1992, hail damaged 18 aircraft at Mineralnye Vody Airport.
Hazardous atmospheric conditions include lightning. They kill people, livestock, cause fires, damage the power grid. About 10,000 people die from thunderstorms and their consequences every year in the world. Moreover, in some regions of Africa, in France and the USA, the number of victims from lightning is greater than from other natural phenomena. Annual economic damage from thunderstorms in the United States is at least $ 700 million.
Droughts are characteristic of desert, steppe and forest-steppe regions. The lack of precipitation causes drying of the soil, lowering the level of groundwater and in water bodies until they are completely dry. The lack of moisture leads to the death of vegetation and crops. Droughts are particularly severe in Africa, the Middle East, Central Asia and southern North America.
Droughts change the human condition, have an adverse effect on the natural environment through such processes as salinization of the soil, dry winds, dust storms, soil erosion and forest fires. Especially strong fires occur during droughts in taiga areas, tropical and subtropical forests and savannas.
Droughts are short-term processes that continue for one season. In the case when droughts last more than two seasons, there is a threat of famine and mass mortality. Usually the effect of drought spreads over the territory of one or more countries. Especially often prolonged droughts with tragic consequences occur in the Sahel region of Africa.
Atmospheric phenomena such as snowfall, short rain showers and prolonged prolonged rains bring great damage. Snowfalls cause massive avalanche creeps in the mountains, and the rapid melting of the snow and heavy rain showers lead to flooding. The huge mass of water falling on the earth's surface, especially in treeless areas, causes severe erosion of the soil cover. There is an intensive growth of gully systems. Floods occur as a result of large floods during a period of heavy precipitation or high water after a sudden warming or spring thawing of snow and, therefore, by origin are related to atmospheric phenomena (they are discussed in the chapter on the ecological role of the hydrosphere).
Anthropogenic atmospheric changes
Currently, there are many different anthropogenic sources that cause air pollution and lead to serious ecological imbalances. The scale of the greatest impact on the atmosphere have two sources: transport and industry. On average, the share of transport accounts for about 60% of the total amount of atmospheric pollution, industry - 15, thermal energy - 15, technologies for the destruction of household and industrial waste - 10%.
Transport, depending on the fuel used and the types of oxidizing agents, releases into the atmosphere oxides of nitrogen, sulfur, oxides and carbon dioxide, lead and its compounds, carbon black, benzopyrene (a substance from the group of polycyclic aromatic hydrocarbons, which is a strong carcinogen causing skin cancer).
The industry emits sulfur dioxide, carbon oxides and dioxide, hydrocarbons, ammonia, hydrogen sulfide, sulfuric acid, phenol, chlorine, fluorine and other compounds and chemical elements into the atmosphere. But the dominant position among emissions (up to 85%) is dust.
As a result of pollution, the transparency of the atmosphere changes, aerosols occur in it, smog and acid rain.
Aerosols are dispersed systems consisting of solid particles or liquid droplets suspended in a gaseous medium. The particle size of the dispersed phase is usually 10 -3 -10 -7 cm. Depending on the composition of the dispersed phase, aerosols are divided into two groups. One of them is aerosols consisting of solid particles dispersed in a gaseous medium, the second is aerosols, which are a mixture of gaseous and liquid phases. The first is called smoke, and the second - fog. In the process of their formation, condensation centers play an important role. Volcanic ash, cosmic dust, products of industrial emissions, various bacteria, etc. act as condensation nuclei. The number of possible sources of concentration nuclei is constantly growing. For example, when dry grass is destroyed by fire on an area of 4000 m 2, an average of 11 * 10 22 aerosol nuclei is formed.
Aerosols began to form from the moment of the emergence of our planet and influenced natural conditions. However, their number and actions, being balanced with the general circulation of substances in nature, did not cause profound environmental changes. The anthropogenic factors of their formation shifted this equilibrium towards significant biospheric overloads. This feature is especially pronounced since the mankind began to use specially created aerosols both in the form of toxic substances and for the protection of plants.
The most dangerous for vegetation are aerosols of sulfur dioxide, hydrogen fluoride and nitrogen. In contact with the wet surface of the sheet, they form acids, detrimental effects on living tissue. Acid mists get along with inhaled air into the respiratory organs of animals and humans, aggressively affect the mucous membranes. Some of them decompose living tissue, and radioactive aerosols cause cancer. Among radioactive isotopes, S 90 represents a particular danger not only because of its carcinogenicity, but also as an analog of calcium, replacing it in the bones of organisms, causing their decomposition.
During nuclear explosions, radioactive aerosol clouds form in the atmosphere. Small particles with a radius of 1 - 10 microns fall not only into the upper layers of the troposphere, but also into the stratosphere, in which they are able to exist for a long time. Aerosol clouds are also formed during the operation of reactors of industrial plants producing nuclear fuel, as well as as a result of accidents at nuclear power plants.
Smog is a mixture of aerosols with liquid and solid dispersed phases, which form a hazy curtain over industrial areas and large cities.
There are three types of smog: icy, wet and dry. Ice smog is called Alaskan. This is a combination of gaseous pollutants with the addition of dust particles and ice crystals, which occur when the droplets of mist and steam heating systems freeze.
Wet smog, or London-type smog, is sometimes called winter. It is a mixture of gaseous pollutants (mainly sulfurous anhydrite), dust particles and mist droplets. A meteorological prerequisite for the appearance of winter smog is windless weather, in which a layer of warm air is located above the surface layer of cold air (below 700 m). In this case, there is not only horizontal, but also vertical exchange. Contaminants, usually dispersed in the high layers, in this case accumulate in the surface layer.
Dry smog occurs in the summer, and it is often called the Los Angeles-type smog. It is a mixture of ozone, carbon monoxide, nitrogen oxides and acid vapors. This smog is formed as a result of the decomposition of pollutants by solar radiation, especially the ultraviolet part of it. A meteorological prerequisite is atmospheric inversion, manifested in the appearance of a layer of cold air over warm. Usually, gases and solid particles raised by warm air flows are then scattered in the upper cold layers, but in this case they accumulate in the inversion layer. In the process of photolysis, nitrogen oxides formed during the combustion of fuel in car engines break down:
NO 2 → NO + O
Then ozone synthesis occurs:
O + O 2 + M → O 3 + M
NO + O → NO 2
Photodissociation processes are accompanied by a yellow-green glow.
In addition, reactions occur as follows: SO 3 + H 2 0 -\u003e H 2 SO 4, i.e., strong sulfuric acid is formed.
With the change in meteorological conditions (the appearance of wind or a change in humidity) cold air dissipates and smog disappears.
The presence of carcinogenic substances in smog leads to respiratory failure, irritation of the mucous membranes, circulatory disorders, asthmatic suffocation and often death. Could especially dangerous for young children.
Acid rain is precipitation acidified by industrial emissions of sulfur oxides, nitrogen and perchloric acid vapors and chlorine dissolved in them. In the process of burning coal, oil and gas, most of the sulfur in it, both in the form of oxide and in compounds with iron, in particular in pyrite, pyrrhotite, chalcopyrite, etc., is converted into sulfur oxide, which, together with carbon dioxide, is released in atmosphere. When atmospheric nitrogen and technical emissions are combined with oxygen, various nitrogen oxides are formed, and the volume of nitrogen oxides formed depends on the combustion temperature. The bulk of the nitrogen oxides occurs during the operation of vehicles and diesel locomotives, and a smaller part falls on energy and industrial enterprises. Sulfur and nitrogen oxides are the main acid-forming agents. When reacting with atmospheric oxygen and water vapor in it, sulfuric and nitric acids are formed.
It is known that the alkaline acid balance of the medium is determined by the pH value. The neutral medium has a pH value equal to 7, acidic - 0, and alkaline - 14 (Fig. 6.7). In the modern era, the pH of rainwater is 5.6, although in the recent past it was neutral. Reducing the pH value by one corresponds to a tenfold increase in acidity and, therefore, at present almost all rains with high acidity occur. The maximum acidity of rain recorded in Western Europe was 4–3.5 pH. It should be noted that the pH value of 4-4.5 is lethal for most fish.
Acid rain has an aggressive effect on the vegetation cover of the Earth, on industrial and residential buildings, and contributes to a significant acceleration of weathering of exposed rocks. An increase in acidity prevents self-regulation of the neutralization of soils in which nutrients dissolve. In turn, this leads to a sharp decline in yield and causes degradation of vegetation cover. The acidity of the soil contributes to the release of heavy metals in a bound state, which are gradually absorbed by plants, causing them serious tissue damage and penetrating into human food chains.
Changes in the alkaline acid potential of marine waters, especially in shallow waters, lead to the cessation of reproduction of many invertebrates, cause fish death and disrupt the ecological balance in the oceans.
As a result of acid rain, the forests of Western Europe, the Baltic States, Karelia, the Urals, Siberia and Canada are under threat of death.
Earth's atmosphere
Atmosphere (from. dr.-greek ἀτμός - steam and σφαῖρα - ball) - gas shell ( geosphere) surrounding the planet Land. Its inner surface covers hydrosphere and partially bark, external borders on the near-Earth part of outer space.
The set of sections of physics and chemistry, studying the atmosphere, is called atmospheric physics. The atmosphere determines weather on the surface of the earth studying weather meteorologyand long variations climate - climatology.
Atmosphere structure
Atmosphere structure
Troposphere
Its upper limit is at an altitude of 8-10 km in the polar, 10-12 km in temperate and 16-18 km in tropical latitudes; In winter, lower than in summer. The lower, the main layer of the atmosphere. Contains more than 80% of the total mass of atmospheric air and about 90% of all water vapor in the atmosphere. The troposphere is highly developed. turbulence and convection, arise the cloudsare developing cyclones and anticyclones. The temperature decreases with increasing height with an average vertical gradient 0.65 ° / 100 m
The following “normal conditions” at the Earth’s surface are taken: density 1.2 kg / m3, barometric pressure 101.35 kPa, temperature plus 20 ° C and relative humidity 50%. These conventional indicators have a purely engineering value.
Stratosphere
The layer of the atmosphere, located at an altitude of 11 to 50 km. Characterized by a slight change in temperature in the layer of 11-25 km (the lower layer of the stratosphere) and its increase in the layer of 25-40 km from −56.5 to 0.8 ° WITH (upper stratosphere or region inversion). Reaching at an altitude of about 40 km, a value of about 273 K (almost 0 ° C), the temperature remains constant to an altitude of about 55 km. This area of constant temperature is called stratopause and is the boundary between the stratosphere and mesosphere.
Stratopause
The boundary layer of the atmosphere between the stratosphere and the mesosphere. In the vertical temperature distribution, a maximum occurs (about 0 ° C).
Mesosphere
Earth's atmosphere
Mesosphere starts at an altitude of 50 km and extends to 80-90 km. The temperature decreases with a height with an average vertical gradient of (0.25-0.3) ° / 100 m. The main energy process is radiant heat transfer. Complex photochemical processes involving free radicals, vibrationally excited molecules, etc., cause the atmosphere to glow.
Mesopause
The transition layer between the mesosphere and the thermosphere. In the vertical temperature distribution, there is a minimum (about -90 ° C).
Karman line
Altitude above sea level, which is conditionally accepted as the boundary between the Earth’s atmosphere and space.
Thermosphere
Main article: Thermosphere
The upper limit is about 800 km. The temperature rises to altitudes of 200-300 km, where it reaches values of the order of 1500 K, after which it remains almost constant to high altitudes. Under the action of ultraviolet and x-ray solar radiation and cosmic radiation, air is ionized (" auroras") - main areas ionosphere lie inside the thermosphere. At altitudes above 300 km atomic oxygen prevails.
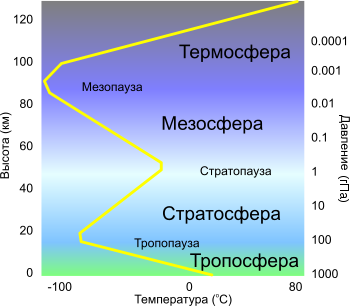
Atmospheric layers up to a height of 120 km
Exosphere (sphere of dispersion)
Exosphere - the scattering zone, the outer part of the thermosphere, located above 700 km. The gas in the exosphere is highly diluted, and hence the leakage of its particles into interplanetary space ( dissipation).
Up to an altitude of 100 km, the atmosphere is a homogeneous well-mixed mixture of gases. In the higher layers, the height distribution of gases depends on their molecular masses; the concentration of heavier gases decreases faster with distance from the Earth's surface. Due to a decrease in the density of gases, the temperature decreases from 0 ° C in the stratosphere to −110 ° C in the mesosphere. However, the kinetic energy of individual particles at altitudes of 200-250 km corresponds to a temperature of ~ 1500 ° C. Above 200 km, significant fluctuations in temperature and gas density in time and space are observed.
At an altitude of about 2000-3000 km, the exosphere gradually turns into the so-called near space vacuumwhich is filled with highly rarefied interplanetary gas particles, mainly hydrogen atoms. But this gas is only a part of interplanetary matter. The other part consists of dust particles of cometary and meteoric origin. In addition to extremely rarefied dust particles, electromagnetic and corpuscular radiation of solar and galactic origin penetrates into this space.
The share of the troposphere accounts for about 80% of the mass of the atmosphere, the share of the stratosphere - about 20%; the mass of the mesosphere is no more than 0.3%, the thermosphere is less than 0.05% of the total mass of the atmosphere. Based on the electrical properties in the atmosphere, the neutrosphere and ionosphere are emitted. At present, it is believed that the atmosphere extends to an altitude of 2000-3000 km.
Depending on the composition of the gas in the atmosphere emit homosphere and heterosphere. Hetero sphere - This is an area where gravity affects the separation of gases, since their mixing at this height is negligible. Hence the variable composition of the heterosphere. Below it lies a well-mixed, homogeneous composition of the atmosphere, called homosphere. The boundary between these layers is called turbo pauseIt lies at an altitude of about 120 km.
Physical properties
The thickness of the atmosphere - about 2000 - 3000 km from the Earth's surface. Total mass of air - (5.1-5.3) × 10 18 kg. Molar mass clean dry air is 28.966. Pressure at 0 ° C at sea level 101,325 kPa; critical temperature 140.7 ° C; critical pressure of 3.7 MPa; C p 1.0048 × 10 3 J / (kg · K) (at 0 ° C), C v 0.7159 × 10 3 J / (kg · K) (at 0 ° C). The solubility of air in water at 0 ° C is 0.036%, at 25 ° C it is 0.22%.
Physiological and other properties of the atmosphere
Already at an altitude of 5 km above sea level, an untrained person appears oxygen starvation and without adaptation, human performance is significantly reduced. Here ends the physiological zone of the atmosphere. A person’s breathing becomes impossible at an altitude of 15 km, although up to about 115 km the atmosphere contains oxygen.
The atmosphere provides us with oxygen for breathing. However, due to the fall in the total pressure of the atmosphere as it rises to a height, the partial pressure of oxygen decreases accordingly.
In the human lungs constantly contains about 3 liters of alveolar air. Partial pressure oxygen in the alveolar air at normal atmospheric pressure is 110 mm Hg. Art., the pressure of carbon dioxide - 40 mm Hg. Art., and water vapor - 47 mm Hg. Art. With increasing altitude, the oxygen pressure drops, and the total pressure of water vapor and carbon dioxide in the lungs remains almost constant - about 87 mm Hg. Art. The supply of oxygen to the lungs is completely stopped when the pressure of the surrounding air becomes equal to this value.
At an altitude of about 19-20 km, the pressure of the atmosphere decreases to 47 mm Hg. Art. Therefore, at this height begins the boiling of water and interstitial fluid in the human body. Outside the pressurized cabin at these heights, death occurs almost instantly. Thus, from the point of view of human physiology, the “cosmos” begins already at an altitude of 15-19 km.
The dense layers of air - the troposphere and stratosphere - protect us from the damaging effects of radiation. With sufficient air dilution, at altitudes of more than 36 km, ionizing radiation - primary cosmic rays; at altitudes of more than 40 km, the ultraviolet part of the solar spectrum is dangerous for humans.
As we ascend to an ever greater height above the Earth’s surface, they gradually weaken and then completely disappear, such familiar phenomena observed in the lower layers of the atmosphere, such as sound propagation, the occurrence of aerodynamic lift and resistance, heat transfer by convection and etc.
In rarefied air layers spread sound turns out to be impossible. Up to altitudes of 60-90 km, it is still possible to use air resistance and lift for controlled aerodynamic flight. But starting with the heights of 100-130 km, the concepts familiar to every pilot numbers M and sound barrier lose their meaning, there passes the conditional Karman line beyond which begins the sphere of purely ballistic flight, which can be controlled only by using reactive forces.
At altitudes above 100 km, the atmosphere is devoid of another remarkable property — the ability to absorb, conduct, and transfer thermal energy by convection (that is, by mixing air). This means that the various elements of the equipment, the equipment of the orbital space station will not be able to be cooled from the outside, as is usually done on an airplane, with the help of air jets and air radiators. At that altitude, as in general in space, the only way to transfer heat is thermal radiation.
Atmospheric composition
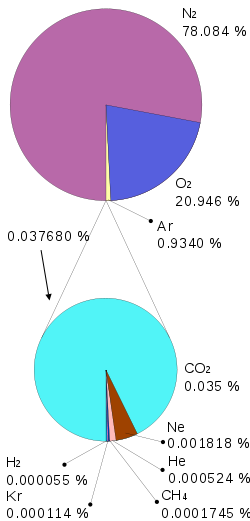
Composition of dry air
The Earth’s atmosphere consists mainly of gases and various impurities (dust, water droplets, ice crystals, sea salts, products of combustion).
The concentration of gases that make up the atmosphere is almost constant, with the exception of water (H 2 O) and carbon dioxide (CO 2).
|
Composition of dry air |
||
|
Nitrogen | ||
|
Oxygen | ||
|
Argon | ||
|
Water | ||
|
Carbon dioxide | ||
|
Neon | ||
|
Helium | ||
|
Methane | ||
|
Krypton | ||
|
Hydrogen | ||
|
Xenon | ||
|
Nitrous oxide | ||
In addition to the gases indicated in the table, the atmosphere contains SO 2, NH 3, CO, ozone, hydrocarbons, HCl, HFcouples HgI 2 as well NO and many other gases in small quantities. In the troposphere there is always a large number of suspended solid and liquid particles ( spray can).
History of the formation of the atmosphere
According to the most common theory, the Earth’s atmosphere was in time in four different compositions. Originally it consisted of light gases ( hydrogen and helium), captured from interplanetary space. This is the so-called primary atmosphere(about four billion years ago). At the next stage, active volcanic activity led to the saturation of the atmosphere with other gases besides hydrogen (carbon dioxide, ammonia, water vapor). So formed secondary atmosphere(about three billion years to this day). This atmosphere was restorative. Further, the process of atmospheric formation was determined by the following factors:
light gas leak (hydrogen and helium) in interplanetary space;
chemical reactions occurring in the atmosphere under the influence of ultraviolet radiation, lightning discharges and some other factors.
Gradually, these factors led to the formation of tertiary atmospherecharacterized by a much lower hydrogen content and a much larger nitrogen and carbon dioxide content (formed from chemical reactions from ammonia and hydrocarbons).
Nitrogen
The formation of a large amount of N 2 is due to the oxidation of the ammonia-hydrogen atmosphere with molecular O 2, which began to come from the surface of the planet as a result of photosynthesis, starting from 3 billion years ago. N 2 is also released into the atmosphere as a result of the denitrification of nitrates and other nitrogen-containing compounds. Nitrogen is oxidized by ozone to NO in the upper atmosphere.
N 2 N reacts only under specific conditions (for example, when lightning discharges). Oxidation of molecular nitrogen by ozone in electrical discharges is used in the industrial manufacture of nitrogen fertilizers. It can be oxidized with low energy consumption and converted into a biologically active form. cyanobacteria (blue-green algae) and nodule bacteria forming rhizobial symbiosis with legumes plants, the so-called. sideratami.
Oxygen
The composition of the atmosphere began to change radically with the advent of the Earth. living organismsas a result photosynthesisaccompanied by the release of oxygen and absorption of carbon dioxide. Initially, oxygen was spent on the oxidation of reduced compounds — ammonia, hydrocarbons, and the acid form. glandcontained in the oceans, etc. At the end of this stage, the oxygen content in the atmosphere began to grow. Gradually formed a modern atmosphere with oxidizing properties. Since it caused serious and dramatic changes in many processes occurring in the atmosphere, lithosphere and biosphereThis event is called Oxygen Catastrophe.
During phanerozoic The composition of the atmosphere and the oxygen content underwent changes. They correlated primarily with the rate of deposition of organic sedimentary rocks. Thus, during periods of coal accumulation, the oxygen content in the atmosphere, apparently, markedly exceeded the current level.
Carbon dioxide
The CO 2 content in the atmosphere depends on the volcanic activity and chemical processes in the earth’s envelopes, but most of all on the intensity of the biosynthesis and decomposition of organic matter in biosphere Of the earth. Virtually the entire current biomass of the planet (about 2.4 × 10 12 tons ) is formed by carbon dioxide, nitrogen and water vapor contained in atmospheric air. Buried in the ocean, at swamps and in the woods organic matter turns into coal, oil and natural gas. (cm. Geochemical carbon cycle)
Noble gases
Inert gas source - argon, helium and krypton - volcanic eruptions and decay of radioactive elements. The earth as a whole and the atmosphere in particular are depleted with inert gases as compared with space. It is believed that the reason for this lies in the continuous leakage of gases into interplanetary space.
Air pollution
Recently, the evolution of the atmosphere began to influence person. The result of his activity was a constant significant increase in the content of carbon dioxide in the atmosphere due to the combustion of hydrocarbon fuels accumulated in previous geological epochs. Huge quantities of CO 2 are consumed during photosynthesis and absorbed by the world's oceans. This gas enters the atmosphere due to the decomposition of carbonate rocks and organic matter of plant and animal origin, as well as due to volcanism and human production. Over the past 100 years, the CO 2 content in the atmosphere has increased by 10%, with the main part (360 billion tons) coming from the combustion of fuel. If the growth rate of fuel combustion continues, in the next 50–60 years the amount of CO 2 in the atmosphere will double and may lead to global climate change.
Fuel combustion - a major source of polluting gases ( WITH, NO, SO 2 ). Sulfur dioxide is oxidized by air oxygen to SO 3 in the upper atmosphere, which in turn interacts with water vapor and ammonia, and the resulting sulfuric acid (H 2 SO 4 ) and ammonium sulfate ((NH 4 ) 2 SO 4 ) return to the surface of the Earth in the form of so-called. acid rain. Using internal combustion engines leads to significant pollution of the atmosphere with nitrogen oxides, hydrocarbons and lead compounds ( tetraethyl lead Pb (CH 3 CH 2 ) 4 ) ).
Aerosol pollution of the atmosphere is due both to natural causes (volcanic eruptions, dust storms, drift of sea water and plant pollen, etc.) and human economic activities (mining of ores and building materials, fuel combustion, cement production, etc.). Intensive large-scale removal of solid particles into the atmosphere is one of the possible causes of climate change on the planet.
The atmosphere is what makes life possible on Earth. The very first information and facts about the atmosphere we get back in primary school. In high school we are already more familiar with this concept in geography class.
The concept of the earth's atmosphere
The atmosphere is present not only on the Earth, but also on other celestial bodies. So called gas envelope surrounding the planet. The composition of this gas layer of different planets is significantly different. Let's take a look at the basics and facts about otherwise called air.
Its most important component is oxygen. Some people mistakenly think that the earth’s atmosphere consists entirely of oxygen, but in reality air is a mixture of gases. It contains 78% nitrogen and 21% oxygen. The remaining one percent includes ozone, argon, carbon dioxide, water vapor. Let the percentage ratio of these gases is small, but they perform an important function - they absorb a significant part of the solar radiant energy, thereby preventing the sun from turning all life on our planet into ashes. Atmospheric properties vary with altitude. For example, at an altitude of 65 km, nitrogen is 86%, and oxygen is 19%.
The composition of the Earth’s atmosphere
- Carbon dioxide necessary for plant nutrition. In the atmosphere it appears as a result of the process of breathing of living organisms, decay, burning. Its absence in the composition of the atmosphere would make the existence of any plant impossible.
- Oxygen - vital for human component of the atmosphere. Its presence is a condition for the existence of all living organisms. It makes up about 20% of the total volume of atmospheric gases.
- Ozone - It is a natural absorber of solar ultraviolet radiation, which adversely affects living organisms. Most of it forms a separate layer of the atmosphere - the ozone screen. Recently, human activity has led to the fact that it is beginning to gradually collapse, but since it is of great importance, then active work is being done on its preservation and restoration.
- Water vapor determines the humidity of the air. Its content may vary depending on various factors: air temperature, location, season. At low temperature, water vapor in the air is very small, maybe less than one percent, and at high temperatures it reaches 4%.
- In addition to all of the above, a certain percentage is always present in the composition of the earth's atmosphere. solid and liquid impurities. These are soot, ash, sea salt, dust, water droplets, microorganisms. They can get into the air both naturally and anthropogenically.
Atmosphere layers
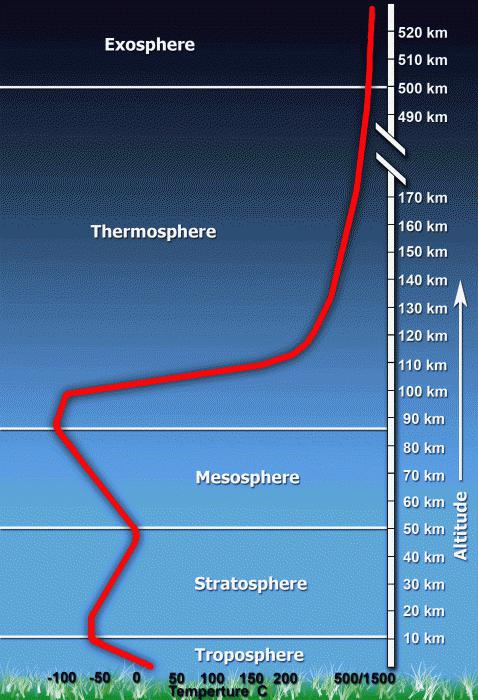
And the temperature, and density, and the qualitative composition of the air varies at different heights. Because of this, it is customary to isolate different layers of the atmosphere. Each of them has its own characteristic. Let's find out which layers of the atmosphere are distinguished:
- Troposphere - this layer of the atmosphere is closest to the surface of the Earth. Its height is 8-10 km above the poles and 16-18 km in the tropics. Here is 90% of all water vapor that is in the atmosphere, therefore, active formation of clouds. Also in this layer are observed such processes as the movement of air (wind), turbulence, convection. The temperature ranges from +45 degrees at noon during the warm season in the tropics to -65 degrees at the poles.
- The stratosphere is the second layer of the atmosphere at the distance from the earth's surface. It is located at an altitude of 11 to 50 km. In the lower layer of the stratosphere, the temperature is approximately -55, in the direction of distance from the Earth, it rises to + 1 ° C. This area is called inversion and is the boundary of the stratosphere and mesosphere.
- The mesosphere is located at an altitude of 50 to 90 km. The temperature at its lower boundary is about 0, at the top it reaches -80 ...- 90 ˚С. Meteorites that enter the atmosphere of the Earth completely burn in the mesosphere, because of this, the air is glowing.
- The thermosphere is approximately 700 km thick. In this layer of the atmosphere auroras appear. They appear due to the action of cosmic radiation and radiation emanating from the sun.
- The exosphere is a zone of air dispersion. Here, the concentration of gases is small and their gradual departure into the interplanetary space takes place.
The boundary between the earth's atmosphere and space expanses is considered to be the milestone of 100 km. This feature is called the Pocket line.
Atmospheric pressure
Listening to the weather forecast, we often hear indicators of atmospheric pressure. But what does atmospheric pressure mean, and how can it affect us?
We figured out that the air consists of gases and impurities. Each of these components has its own weight, which means that the atmosphere is not weightless, as it was believed until the XVII century. Atmospheric pressure is the force with which all layers of the atmosphere press on the surface of the Earth and on all objects.
Scientists have made complex calculations and proved that the atmosphere weighs 10,333 kg per square meter. This means that the human body is subject to air pressure, whose weight is equal to 12-15 tons. Why don't we feel this? It saves us its internal pressure, which balances the external. You can feel the pressure of the atmosphere, while in the plane or high in the mountains, as the atmospheric pressure at a height is much less. At the same time physical discomfort, laying of ears, dizziness is possible.

A lot can be said about the atmosphere around. We know a lot about her interesting facts, and some of them may seem amazing:
- The weight of the earth's atmosphere is 5 300 000 000 000 000 000 tons.
- It contributes to the transmission of sound. At an altitude of more than 100 km, this property disappears due to a change in the composition of the atmosphere.
- The movement of the atmosphere is provoked by the uneven heating of the Earth’s surface.
- A thermometer is used to determine the air temperature, and a barometer is used to determine the pressure force of the atmosphere.
- The presence of the atmosphere saves our planet from 100 tons of meteorites daily.
- The composition of the air was fixed several hundred million years, but began to change with the onset of rapid industrial activity.
- It is believed that the atmosphere extends up to an altitude of 3000 km.
The value of the atmosphere for humans
The physiological zone of the atmosphere is 5 km. At an altitude of 5000 m above sea level, a person begins to manifest itself, which is reflected in a decrease in his working capacity and deterioration of health. This shows that a person cannot survive in a space where this amazing mixture of gases is not.
All information and facts about the atmosphere only confirm its importance to people. Due to its presence, the opportunity to develop life on Earth has appeared. Already today, having assessed the scale of the harm that humanity is capable of causing to life-giving air with its actions, we should think about further measures for the preservation and restoration of the atmosphere.
At sea level 1013.25 hPa (about 760 mm Hg). The average temperature of the globe at the surface of the Earth is 15 ° C, while the temperature varies from about 57 ° C in subtropical deserts to -89 ° C in Antarctica. Air density and pressure decrease with height according to a law close to exponential.
Atmosphere structure. Vertically, the atmosphere has a layered structure, determined mainly by the features of the vertical temperature distribution (figure), which depends on geographic location, season, time of day, and so on. The lower layer of the atmosphere - the troposphere - is characterized by a drop in temperature with a height (approximately 6 ° С per 1 km), its height from 8-10 km in polar latitudes to 16-18 km in the tropics. Due to the rapid decrease in air density with height in the troposphere is about 80% of the total mass of the atmosphere. Above the troposphere is the stratosphere - a layer that is characterized by a general increase in temperature with altitude. The transition layer between the troposphere and the stratosphere is called the tropopause. In the lower stratosphere to a level of about 20 km, the temperature varies little with height (the so-called isothermal region) and often even slightly decreases. Higher temperature increases due to absorption of solar UV radiation by ozone, initially slowly, and from the level of 34-36 km - faster. The upper limit of the stratosphere - the stratopause - is located at an altitude of 50-55 km, corresponding to a maximum temperature (260-270 K). The atmosphere layer, located at an altitude of 55-85 km, where the temperature drops again with altitude, is called the mesosphere, at its upper boundary, the mesopause, the temperature reaches 150-160 K in the summer and 200-230 K in the winter. A thermosphere begins in the mesopause, characterized by a rapid rise in temperature, reaching values of 800-1200 K at an altitude of 250 km. The solar and X-ray radiation of the Sun is absorbed in the thermosphere, meteors are decelerated and burned, so it acts as a protective layer of the Earth. Higher still is the exosphere, from where atmospheric gases are scattered into world space due to dissipation and where there is a gradual transition from the atmosphere to interplanetary space.
Atmospheric composition. Up to an altitude of about 100 km, the atmosphere is almost uniform in chemical composition and the average molecular weight of air (about 29) is constant. Near the Earth’s surface, the atmosphere consists of nitrogen (about 78.1% by volume) and oxygen (about 20.9%), and also contains small amounts of argon, carbon dioxide (carbon dioxide), neon, and other fixed and variable components (see Air ).
In addition, the atmosphere contains small amounts of ozone, nitrogen oxides, ammonia, radon, and others. The relative content of the main components of air is constant over time and uniformly in different geographical areas. The content of water vapor and ozone is variable in space and time; despite their low content, their role in atmospheric processes is very significant.
Above 100–110 km, dissociation of oxygen, carbon dioxide and water vapor occurs, and therefore the molecular weight of air decreases. At an altitude of about 1000 km light gases start to dominate - helium and hydrogen, and even higher the Earth's atmosphere gradually changes into interplanetary gas.
The most important variable component of the atmosphere is water vapor, which enters the atmosphere when it evaporates from the surface of water and moist soil, as well as by transpiration by plants. The relative content of water vapor varies at the earth’s surface from 2.6% in the tropics to 0.2% in polar latitudes. With height, it falls rapidly, decreasing by half already at an altitude of 1.5-2 km. In the vertical column of the atmosphere in temperate latitudes contains about 1.7 cm "layer of precipitated water." During the condensation of water vapor, clouds are formed, from which atmospheric precipitation falls in the form of rain, hail, and snow.
An important component of atmospheric air is ozone, concentrated at 90% in the stratosphere (between 10 and 50 km), about 10% of it is in the troposphere. Ozone provides absorption of hard UV radiation (with a wavelength of less than 290 nm), and this is its protective role for the biosphere. Values of total ozone content vary with latitude and season in the range from 0.22 to 0.45 cm (thickness of the ozone layer at a pressure of p = 1 atm and temperature T = 0 ° C). In the ozone holes observed in spring in Antarctica since the early 1980s, the ozone content can fall to 0.07 cm. It increases from the equator to the poles and has an annual course with a maximum in spring and a minimum in the autumn, and the amplitude of the annual course is small in the tropics and grows to high latitudes. A significant variable component of the atmosphere is carbon dioxide, whose content in the atmosphere over the past 200 years has increased by 35%, which is mainly due to anthropogenic factors. Its latitudinal and seasonal variability associated with photosynthesis of plants and solubility in seawater is observed (according to Henry's law, the solubility of gas in water decreases with increasing its temperature).
An important role in shaping the climate of the planet is played by atmospheric aerosol - solid and liquid particles suspended in air, ranging in size from a few nm to tens of microns. Different aerosols of natural and anthropogenic origin. The aerosol is formed in the process of gas-phase reactions from plant waste products and human economic activities, volcanic eruptions, as a result of dust rising by the wind from the surface of the planet, especially from its desert regions, and also formed from cosmic dust entering the upper layers of the atmosphere. Most of the aerosol is concentrated in the troposphere, the aerosol from volcanic eruptions forms the so-called Junge layer at an altitude of about 20 km. The greatest amount of anthropogenic aerosol enters the atmosphere as a result of vehicles and CHP, chemical plants, fuel combustion, etc. Therefore, in some areas, the composition of the atmosphere is noticeably different from ordinary air, which required the creation of a special service of observation and monitoring the level of air pollution.
Evolution of the atmosphere. The modern atmosphere seems to be of secondary origin: it was formed from gases emitted by the solid shell of the Earth after the completion of the formation of the planet about 4.5 billion years ago. During the geological history of the Earth, the atmosphere has undergone significant changes in its composition under the influence of a number of factors: dissipation (volatilization) of gases, mostly lighter, into outer space; gas emissions from the lithosphere as a result of volcanic activity; chemical reactions between the components of the atmosphere and the rocks composing the crust; photochemical reactions in the atmosphere itself under the influence of solar UV radiation; accretion (capture) of the interplanetary medium matter (for example, meteoric matter). The development of the atmosphere is closely connected with geological and geochemical processes, and the last 3-4 billion years also with the activity of the biosphere. A significant part of the gases that make up the modern atmosphere (nitrogen, carbon dioxide, water vapor), arose in the course of volcanic activity and intrusion, which carried them from the depths of the Earth. Oxygen appeared in significant quantities about 2 billion years ago as a result of the activity of photosynthetic organisms originally originated in the surface waters of the ocean.
According to the data on the chemical composition of carbonate sediments, estimates of the amount of carbon dioxide and oxygen in the atmosphere of the geological past have been obtained. Over the Phanerozoic (the last 570 million years of Earth’s history), the amount of carbon dioxide in the atmosphere varied widely according to the level of volcanic activity, ocean temperature and the level of photosynthesis. For most of this time, the concentration of carbon dioxide in the atmosphere was significantly higher (up to 10 times). The amount of oxygen in the atmosphere of the Phanerozoic significantly changed, and the tendency to its increase prevailed. In the atmosphere of the Precambrian, the mass of carbon dioxide was, as a rule, greater, and the mass of oxygen, less than the atmosphere of the Phanerozoic. The fluctuations in the amount of carbon dioxide in the past had a significant effect on climate, increasing the greenhouse effect with an increase in carbon dioxide concentration, due to which the climate throughout the main part of the Phanerozoic was much warmer compared to the modern era.
Atmosphere and life. Without atmosphere, Earth would be a dead planet. Organic life proceeds in close cooperation with the atmosphere and its associated climate and weather. Insignificant in mass compared to the planet as a whole (approximately one millionth part), the atmosphere is an indispensable condition for all forms of life. The greatest value of atmospheric gases for the life of organisms are oxygen, nitrogen, water vapor, carbon dioxide, ozone. When carbon dioxide is absorbed by photosynthetic plants, organic matter is created, which is used as an energy source by the vast majority of living beings, including humans. Oxygen is necessary for the existence of aerobic organisms, for which the flow of energy is provided by the oxidation reactions of organic matter. Nitrogen assimilated by some microorganisms (nitrogen fixers) is necessary for mineral nutrition of plants. Ozone, which absorbs the hard UV radiation of the Sun, significantly reduces this harmful part of the life of solar radiation. Condensation of water vapor in the atmosphere, the formation of clouds and the subsequent precipitation of water supply water to the land, without which no life forms are possible. The vital activity of organisms in the hydrosphere is largely determined by the amount and chemical composition of atmospheric gases dissolved in water. Since the chemical composition of the atmosphere essentially depends on the activity of organisms, the biosphere and atmosphere can be considered as part of a single system, the maintenance and evolution of which (see Biogeochemical cycles) was of great importance for changing the composition of the atmosphere throughout the Earth’s history as a planet.
Radiation, heat and water balance of the atmosphere. Solar radiation is practically the only source of energy for all physical processes in the atmosphere. main feature radiation regime atmosphere - the so-called greenhouse effect: the atmosphere sufficiently transmits solar radiation to the earth’s surface, but actively absorbs thermal long-wave radiation from the earth’s surface, part of which returns to the surface in the form of counter-radiation, compensating for the radiative heat loss of the earth’s surface (see Atmospheric Radiation). In the absence of an atmosphere, the average temperature of the earth’s surface would be -18 ° C, in fact it is 15 ° C. The incoming solar radiation is partially (about 20%) absorbed into the atmosphere (mainly water vapor, water droplets, carbon dioxide, ozone and aerosols), and is also scattered (about 7%) on aerosol particles and density fluctuations (Rayleigh scattering). The total radiation, reaching the earth's surface, is partially (about 23%) reflected from it. The reflection coefficient is determined by the reflectivity of the underlying surface, the so-called albedo. On average, the Earth’s albedo for the integral solar radiation flux is close to 30%. It varies from a few percent (dry soil and chernozem) to 70-90% for fresh snow. The radiative heat exchange between the earth's surface and the atmosphere essentially depends on the albedo and is determined by the effective radiation of the Earth’s surface and the counter-radiation of the atmosphere absorbed by it. The algebraic sum of radiation fluxes entering the earth’s atmosphere from outer space and leaving it back is called the radiation balance.
 Transformations of solar radiation after it is absorbed by the atmosphere and the earth’s surface determine the heat balance of the Earth as a planet. The main source of heat for the atmosphere is the earth's surface; the heat from it is transmitted not only in the form of long-wave radiation, but also by convection, and is also released during condensation of water vapor. The shares of these inflows of heat are on average 20%, 7% and 23%, respectively. It also adds about 20% of heat due to the absorption of direct solar radiation. The solar radiation flux per unit of time across a single area perpendicular to the sun’s rays and located outside the atmosphere at an average distance from the Earth to the Sun (the so-called solar constant) is 1367 W / m 2, the changes are 1-2 W / m 2 depending on cycle solar activity. With a planetary albedo about 30% of the time average global solar energy inflow to the planet is 239 W / m 2. Since the Earth as a planet emits into space an average of the same amount of energy, then, according to the Stefan-Boltzmann law, the effective temperature of the outgoing thermal long-wave radiation is 255 K (-18 ° C). At the same time, the average temperature of the earth's surface is 15 ° C. The difference of 33 ° C occurs due to greenhouse effect.
Transformations of solar radiation after it is absorbed by the atmosphere and the earth’s surface determine the heat balance of the Earth as a planet. The main source of heat for the atmosphere is the earth's surface; the heat from it is transmitted not only in the form of long-wave radiation, but also by convection, and is also released during condensation of water vapor. The shares of these inflows of heat are on average 20%, 7% and 23%, respectively. It also adds about 20% of heat due to the absorption of direct solar radiation. The solar radiation flux per unit of time across a single area perpendicular to the sun’s rays and located outside the atmosphere at an average distance from the Earth to the Sun (the so-called solar constant) is 1367 W / m 2, the changes are 1-2 W / m 2 depending on cycle solar activity. With a planetary albedo about 30% of the time average global solar energy inflow to the planet is 239 W / m 2. Since the Earth as a planet emits into space an average of the same amount of energy, then, according to the Stefan-Boltzmann law, the effective temperature of the outgoing thermal long-wave radiation is 255 K (-18 ° C). At the same time, the average temperature of the earth's surface is 15 ° C. The difference of 33 ° C occurs due to greenhouse effect.
The water balance of the atmosphere as a whole corresponds to the equality of the amount of moisture evaporated from the surface of the Earth, the amount of precipitation falling on the earth's surface. The atmosphere over the oceans receives more moisture from the processes of evaporation than over land, and loses in the form of precipitation 90%. Excess water vapor over the oceans is transported to the continents by air currents. The amount of water vapor transported to the atmosphere from the oceans to the continents is equal to the volume of flow of the rivers flowing into the oceans.
Air movement. Earth has a spherical shape, therefore, to its high latitudes comes much less solar radiation than the tropics. As a result, large temperature contrasts appear between the latitudes. Temperature distribution is also significantly affected by the relative position of the oceans and continents. Due to the large mass of oceanic waters and the high heat capacity of the water, seasonal variations in ocean surface temperature are much less than land. In this connection, in the middle and high latitudes, the air temperature over the oceans is noticeably lower in summer than over continents, and higher in winter.
The uneven heating of the atmosphere in different regions of the globe causes a non-uniform spatial distribution of atmospheric pressure. At sea level, the pressure distribution is characterized by relatively low values near the equator, an increase in subtropics (high pressure belts) and a decrease in middle and high latitudes. At the same time, the pressure in winter over the continents of extratropical latitudes is usually increased, and in summer it is lowered, due to the temperature distribution. Under the action of a pressure gradient, air experiences an acceleration directed from high pressure areas to low areas, which leads to movement of air masses. The moving air masses are also affected by the deflecting force of the Earth’s rotation (Coriolis force), the frictional force decreasing with height, and with curved trajectories and centrifugal force. Of great importance is turbulent air mixing (see Turbulence in the atmosphere).
A complex system of air currents (general circulation of the atmosphere) is associated with the planetary pressure distribution. In the meridional plane, on average, two or three cells of the meridional circulation can be traced. Near the equator, heated air rises and falls in the subtropics, forming the Hadley cell. In the same place air of the return cell of Ferrell falls. In high latitudes, a straight polar cell is often traced. The speed of the meridional circulation is about 1 m / s or less. Due to the effect of Coriolis force, westerly winds are observed in most of the atmosphere with velocities in the middle troposphere of about 15 m / s. There are relatively stable wind systems. These include trade winds - winds blowing from high-pressure belts in the subtropics to the equator with a noticeable eastern component (from east to west). Monsoons are fairly stable - air currents that are clearly seasonal in nature: they blow from the ocean to the mainland in summer and in the opposite direction in winter. The Indian Ocean monsoons are especially regular. In mid-latitudes, the movement of air masses has mainly a westerly direction (from west to east). This is a zone of atmospheric fronts on which large eddies arise - cyclones and anticyclones, covering many hundreds and even thousands of kilometers. Cyclones appear in the tropics; here they are distinguished by smaller sizes, but very high wind speeds reaching hurricane forces (33 m / s and more), so-called tropical cyclones. They are called hurricanes in the Atlantic and Eastern Pacific, and typhoons in the Western Pacific. In the upper troposphere and lower stratosphere in areas separating the direct cell of the Hadley meridional circulation and the return Ferrell cell, there are often relatively narrow, hundreds of kilometers wide, jet streams with sharply delineated boundaries, within which the wind reaches 100-150 and even 200 m / with.
Climate and weather. The difference in the amount of solar radiation, which comes at different latitudes to the Earth’s surface with a variety of physical properties, determines the diversity of the Earth’s climates. From the equator to tropical latitudes, the air temperature at the earth's surface averages 25–30 ° C and varies little throughout the year. In the equatorial belt, usually a lot of precipitation falls, which creates conditions of excessive moisture there. In tropical zones, the amount of precipitation decreases and in some areas becomes very small. Here are the vast deserts of the Earth.
In subtropical and middle latitudes, air temperature varies significantly throughout the year, and the difference between summer and winter temperatures is especially great in regions of the continents remote from the oceans. Thus, in some regions of Eastern Siberia, the annual amplitude of air temperature reaches 65 ° С. The conditions of humidification in these latitudes are very diverse, depend mainly on the mode of general circulation of the atmosphere and vary significantly from year to year.
In polar latitudes, the temperature remains low throughout the year, even if it has a noticeable seasonal variation. This contributes to the wide distribution of ice cover on the oceans and land and permafrost, which occupy over 65% of its area in Russia, mainly in Siberia.
Changes have become more pronounced over the past decades. global climate. Temperature rises more at high latitudes than at low ones; more in winter than in summer; more at night than during the day. Over the 20th century, the average annual air temperature at the earth's surface in Russia has increased by 1.5–2 ° C, and in some regions of Siberia an increase of several degrees has been observed. This is associated with an increase in the greenhouse effect due to an increase in the concentration of small gas impurities.
The weather is determined by the conditions of atmospheric circulation and the geographical location of the area, it is most stable in the tropics and most variable in middle and high latitudes. Most of all, the weather changes in the zones of change of air masses caused by the passage of atmospheric fronts, cyclones and anticyclones carrying precipitation and wind intensification. Data for weather forecasting is collected at ground weather stations, ships and aircraft, from meteorological satellites. See also Meteorology.
Optical, acoustic and electrical phenomena in the atmosphere. When electromagnetic radiation propagates in the atmosphere as a result of refraction, absorption and scattering of light by air and various particles (aerosol, ice crystals, water droplets), various optical phenomena occur: rainbow, crowns, halo, mirage, etc. Light scattering causes the visible height of the heavenly arch and blue sky color The visibility range of objects is determined by the conditions for the propagation of light in the atmosphere (see Atmospheric visibility). From the transparency of the atmosphere at different wavelengths depend on the communication distance and the ability to detect objects with instruments, including the possibility of astronomical observations from the surface of the Earth. The phenomenon of twilight plays an important role in studies of the optical inhomogeneities of the stratosphere and mesosphere. For example, photographing twilight from spacecraft can detect aerosol layers. The features of electromagnetic radiation propagation in the atmosphere determine the accuracy of remote sensing methods for its parameters. All these questions, like many others, are studied by atmospheric optics. Refraction and scattering of radio waves determine the possibility of radio reception (see Radio propagation).
The sound propagation in the atmosphere depends on the spatial distribution of temperature and wind speed (see Atmospheric acoustics). It is of interest for remote sensing of the atmosphere. The explosions of charges launched by rockets into the upper atmosphere gave rich information about the wind systems and the course of temperature in the stratosphere and mesosphere. In a stably stratified atmosphere, when the temperature drops with a height slower than the adiabatic gradient (9.8 K / km), so-called internal waves arise. These waves can propagate upward into the stratosphere and even into the mesosphere, where they attenuate, contributing to increased wind and turbulence.
The negative charge of the Earth and the electric field caused by it, together with the electrically charged ionosphere and magnetosphere, create a global electrical circuit. An important role is played by the formation of clouds and thunderstorm electricity. The danger of lightning discharges has caused the need to develop methods for lightning protection of buildings, structures, power lines and communications. This phenomenon is especially dangerous for aviation. Thunderstorm discharges cause atmospheric radio interference, known as atmospherics (see Whistling atmospherics). During a sharp increase in the electric field intensity, luminous discharges are observed, appearing on the tips and sharp corners of objects protruding above the earth's surface, on separate peaks in the mountains, etc. (Elma lights). The atmosphere always contains a number of light and heavy ions that vary greatly depending on the specific conditions, which determine the electrical conductivity of the atmosphere. The main air ionizers at the earth’s surface are the radiation of radioactive substances contained in the earth’s crust and in the atmosphere, as well as cosmic rays. See also Atmospheric electricity.
The influence of man on the atmosphere. Over the past centuries, there has been an increase in the concentration of greenhouse gases in the atmosphere as a result of human activities. The percentage of carbon dioxide increased from 2.8-10 2 two hundred years ago to 3.8-10 2 in 2005, the methane content increased from 0.7-10 1 approximately 300-400 years ago to 1.8-10 -4 at the beginning of the 21st century; about 20% of the increase in the greenhouse effect over the past century was given by freons, which were practically not present in the atmosphere until the mid-20th century. These substances are recognized as stratospheric ozone destroyers, and their production is prohibited by the 1987 Montreal Protocol. The increasing concentration of carbon dioxide in the atmosphere is caused by the burning of ever-increasing amounts of coal, oil, gas and other types of carbon fuels, as well as by the reduction of forests, which results in a decrease in carbon dioxide absorption through photosynthesis. The concentration of methane increases with the growth of oil and gas production (due to its loss), as well as with the expansion of rice crops and an increase in the number of cattle. All this contributes to climate warming.
To change the weather developed methods of active influence on atmospheric processes. They are used to protect agricultural plants from hail by dispersing special reagents in thunderstorm clouds. There are also methods for dispersing fogs at airports, protecting plants from frost, influencing clouds in order to increase precipitation in the right places, or to disperse clouds at public events.
Exploring the atmosphere. Information on physical processes in the atmosphere is obtained primarily from meteorological observations, which are carried out by a global network of permanently operating meteorological stations and posts located on all continents and on many islands. Daily observations provide information about air temperature and humidity, atmospheric pressure and precipitation, cloudiness, wind, etc. Observations of solar radiation and its transformations are carried out at actinometric stations. Of great importance for the study of the atmosphere are networks of upper-air stations, at which meteorological measurements are made using radiosondes to an altitude of 30-35 km. At a number of stations, observations are made of atmospheric ozone, electrical phenomena in the atmosphere, and the chemical composition of the air.
Data from ground stations are complemented by observations on the oceans, where "weather vessels" operate, which are constantly located in certain areas of the oceans, as well as meteorological information obtained from research and other vessels.
In recent decades, an increasing amount of information about the atmosphere has been obtained with the help of meteorological satellites, on which instruments are installed for photographing clouds and measuring the flux of ultraviolet, infrared and microwave radiation from the sun. Satellites provide information on vertical profiles of temperature, clouds and its water storage, elements of the atmospheric radiation balance, ocean surface temperature, etc. Using measurements of radio signal refraction from a navigation satellite system, it is possible to determine vertical profiles of density, pressure and temperature in the atmosphere, as well as moisture content . With the help of satellites, it became possible to clarify the value of the solar constant and planetary albedo of the Earth, build maps of the radiation balance of the Earth-atmosphere system, measure the content and variability of small atmospheric impurities, and solve many other problems of atmospheric physics and environmental monitoring.
Lit .: Budyko M. I. Climate in the past and the future. L., 1980; Matveev L. T. The course of general meteorology. Physics of the atmosphere. 2nd ed. L., 1984; Budyko M. I., Ronov A. B., Yanshin A. L. History of the atmosphere. L., 1985; Khrgian A. Kh. Atmospheric Physics. M., 1986; Atmosphere: Reference. L., 1991; Khromov S.P., Petrosyants M.A. Meteorology and Climatology. 5th ed. M., 2001.
G. S. Golitsyn, N. A. Zaitsev.
The atmosphere is the gas shell of the Earth with aerosol particles contained in it, moving together with the Earth in world space as a whole and at the same time taking part in the rotation of the Earth. At the bottom of the atmosphere, our life basically flows.
Almost all planets of our solar system possess their atmospheres, but only the terrestrial atmosphere is capable of supporting life.
When our planet was formed 4.5 billion years ago, then, apparently, it was deprived of the atmosphere. The atmosphere was formed as a result of volcanic emissions of water vapor with impurities of carbon dioxide, nitrogen and other chemicals from the bowels of the young planet. But the atmosphere may contain a limited amount of moisture, so its excess as a result of condensation gave rise to the oceans. But then the atmosphere was deprived of oxygen. The first living organisms, which originated and developed in the ocean, as a result of the photosynthesis reaction (H 2 O + CO 2 = CH 2 O + O 2) began to release small portions of oxygen, which began to enter the atmosphere.
The formation of oxygen in the Earth’s atmosphere led to the formation of an ozone layer at altitudes of about 8–30 km. And, thereby, our planet has acquired protection from the damaging effects of ultraviolet research. This circumstance was the impetus for the further evolution of life forms on Earth, because as a result of the enhancement of photosynthesis, the amount of oxygen in the atmosphere began to grow rapidly, which contributed to the formation and maintenance of life forms including on land.
Today our atmosphere is 78.1% nitrogen, 21% oxygen, 0.9% argon, 0.04% carbon dioxide. Compared to the main gases, neon, helium, methane, and krypton constitute very small fractions.
The particles of gas contained in the atmosphere are affected by the force of gravity of the Earth. And, taking into account that the air is compressed, its density gradually decreases with height, passing into outer space without a clear boundary. Half of the entire mass of the earth's atmosphere is concentrated in the lower 5 km, three quarters in the lower 10 km, nine-tenths in the lower 20 km. 99% of the mass of the Earth’s atmosphere is concentrated below an altitude of 30 km, which is only 0.5% of the equatorial radius of our planet.
At sea level, the number of atoms and molecules per cubic centimeter of air is about 2 * 10 19, at an altitude of 600 km only 2 * 10 7. At sea level, an atom or molecule flies about 7 * 10 -6 cm before colliding with another particle. At an altitude of 600 km, this distance is about 10 km. And at sea level, about 7 * 10 9 such collisions occur every second, at an altitude of 600 km - only about one per minute!
But not only pressure changes with height. The temperature is also changing. For example, at the foot of a high mountain it can be quite hot, while the top of the mountain is covered with snow and the temperature there is at the same time below zero. But it is worth climbing the plane to a height of about 10-11 km, as you can hear the message that overboard -50 degrees, while the earth's surface is 60-70 degrees warmer ...
Initially, scientists assumed that the temperature decreases with height until it reaches absolute zero (-273.16 ° C). But it is not.
The Earth's atmosphere consists of four layers: the troposphere, the stratosphere, the mesosphere, the ionosphere (thermosphere). Such a division into layers is taken on the basis of data on temperature change with height. The lowest layer, where the air temperature falls with altitude, was called the troposphere. The layer above the troposphere, where the temperature drop stops, is replaced by isothermal and, finally, the temperature begins to rise, called the stratosphere. The layer above the stratosphere, in which the temperature is rapidly falling again, is the mesosphere. And, finally, the layer where the temperature rises again is called the ionosphere or the thermosphere.
The troposphere extends on average in the lower 12 km. It is here that the formation of our weather takes place. The highest clouds (cirrus) are formed in the uppermost layers of the troposphere. The temperature in the troposphere decreases adiabatically with height, i.e. temperature change occurs due to a decrease in pressure with height. The temperature profile of the troposphere is largely due to solar radiation reaching the Earth’s surface. As a result of the Sun's heating of the Earth’s surface, convective and turbulent flows are formed, directed upward, which form the weather. It is worth noting that the influence of the underlying surface on the lower layers of the troposphere extends to a height of about 1.5 km. Of course, excluding mountain areas.
The upper boundary of the troposphere is the tropopause, the isothermal layer. Recall the characteristic form of thunderstorm clouds, the top of which is the “ejection” of cirrus clouds, called the “anvil”. This “anvil” just “spreads” under the tropopause, since due to isothermia, the ascending air flows are significantly weakened, and the cloud ceases to develop vertically. But in special, rare cases, the tops of cumulonimbus clouds can invade the lower stratosphere, overcoming the tropopause.
The height of the tropopause depends on the latitude. So, at the equator, it is located at an altitude of about 16 km, and its temperature is about -80 ° C. At the poles of the tropopause is located below - approximately at an altitude of 8 km. In summer, its temperature here is -40 ° C, and -60 ° C in winter. Thus, despite more high temperatures at the surface of the Earth, the tropical tropopause is much colder than at the poles.
Further, in the stratosphere, the temperature does not decrease with height, but on the contrary, it grows until it reaches -30 ° C ... + 20 ° C depending on the season and latitude at an altitude of about 48 km. This increase in temperature is due to the interaction of ultraviolet radiation with the ozone layer, which is located just in the stratosphere. By the way, the stratosphere also affects the weather. Recently, studies have emerged that indicate a relationship between the parameters of the stratosphere and surface temperature anomalies. Probably, the development of these studies will allow scientists to develop more advanced and accurate methods for a long-term forecast of temperature anomalies at the Earth's surface (for 30-40 days).
It should be added that the amount of water vapor in the stratosphere decreases sharply, but the ozone content increases. Thus, an obvious contrast is formed between the wet and low ozone-poor troposphere and the dry, but ozone-rich stratosphere.
Despite the dryness of the stratosphere, in the cold season, at high latitudes, clouds can still form in it at altitudes from 17 to 30 km.
The stratosphere extends to about 48 km above the surface of our planet and, together with the troposphere, accounts for 99.9% of our atmosphere.
The upper limit of the stratosphere is the stratopause.
Above the stratopause, the temperature begins to drop again. This layer is called the mesosphere and is located in the middle atmosphere. In the upper layers of the mesosphere, the temperature drops to -90 ° C. Such a beautiful light phenomenon in the atmosphere, like meteor flashes, is born in the mesosphere. Therefore, watching the "falling stars", remember that this phenomenon we see in the mesosphere. Also in the upper layers of the mesosphere, mysterious noctilucent clouds are formed, which in the northern hemisphere of the Earth can be observed on short summer nights from May to August above the northern horizon. The mesosphere ends in a mesopause at an altitude of about 85 km. In high latitudes, the temperature of the mesopause varies from -120 ° C in summer to -50 ° in winter.
In the summer months with an increase in vertical temperature gradients in the mesosphere over high latitudes, incl. Due to the maximum temperature of the stratopause due to the maximum influx of solar radiation, upflows are formed, which lead to the formation of thin clouds, called silver. Noctilucent clouds form in the upper mesosphere at altitudes of about 80 km above the Earth’s surface.
The upper layer of the atmosphere is called the ionosphere (thermosphere). Here, the temperature begins to rise again, and to significant values (up to 500-1000 ° K, depending on solar activity). Daily temperature variations here amount to hundreds of degrees! But the air here is so discharged that the concept of "temperature" in our understanding here means little.
Such beautiful natural phenomena as auroras occur in the ionosphere.
The height of thermopause depending on solar activity varies from 200 to 500 km. Above 500 km, the determination of temperature is a very difficult task due to the extreme rarefaction of these very upper limits of the earth’s atmosphere.